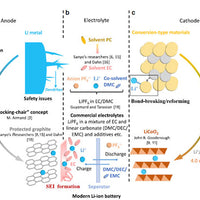
Safety is one of the most concerning issues in the use of powered-type lithium-ion batteries in electric vehicles. There are many influencing factors, including anode and cathode materials, diaphragm, electrolyte, battery design, and power management system, etc. At present, the safety test and evaluation of lithium-ion batteries are all conducted by sampling the finished batteries under different abuse states, and the excellent safety performance of lithium iron phosphate materials and lithium iron phosphate batteries is also tested under these conditions. A more important factor related to the safety of lithium-ion batteries is the possibility and high probability of short circuits due to the material and inherent reasons for the battery. Lithium secondary batteries with lithium metal as the negative electrode are abandoned because of the safety problem of the internal short circuit caused by the occurrence of lithium dendrites piercing the diaphragm during charging and discharging.
Lithium-ion batteries are generally considered safe in normal use, as demonstrated by Toyota's use of nickel compounds, considered the least safe in the industry, as anode materials. Although lithium iron phosphate material has the highest thermal stability and structural stability among all positive electrode materials in terms of thermodynamics and has also been verified in actual safety performance tests, it may be the least safe in terms of the possibility and probability of internal short-circuit of material and battery.
First of all, from material preparation, solid-phase sintering of the lithium iron phosphate is a complex multiphase reaction (although some synthesis technology that is the liquid phase synthesis process, but ultimately the process of sintering in high-temperature solid phase), has a solid phosphate, iron oxide, and lithium salt, plus the precursor and carbon reduction aerosols. To ensure that the iron element in lithium iron phosphate is positive bivalent, the sintering reaction must be carried out in a reducing atmosphere, and in the process of reducing the ferric ion to positive bivalent iron in a strongly reducing atmosphere, it is possible to further reduce the positive bivalent iron to trace elemental iron. Elemental iron is the most taboo substance in batteries, which can cause a micro short circuit. This is one of the important reasons why Lithium iron phosphate has not been used in power lithium-ion batteries in Japan. Also, a significant characteristic of a solid-solid reaction is the slow and incomplete reaction, which makes it possible to have trace amounts of Fe2O3 in lithium iron phosphate. The American Argonauts Laboratory attributed the defect of poor high-temperature cycling of lithium iron phosphate to the dissolution of Fe2O3 in the charging and discharging cycle and the precipitation of elemental iron on the negative pole. Also, to improve the properties of lithium iron phosphate, its particles must be nano-sized. However, a significant feature of nanomaterials is their low structural and thermal stability and high chemical activity, which to some extent also increases the possibility of iron dissolution in lithium iron phosphate, especially under high-temperature cycling and storage conditions. The experimental results also showed that the presence of iron was detected by chemical analysis or energy spectrum analysis on the negative pole.
From the aspects of the preparation of iron phosphate lithium-ion battery, due to the lithium iron phosphate nanoscale particles is small, high specific surface area, and as a result of the carbon coating process, the gas, such as the high specific surface area of activated carbon on the moisture in the air and has a strong adsorption purpose, poor performance caused by electrode processing, binder for poor adhesion of the nanoparticles. During the battery preparation process or the battery charge/discharge cycle and storage, the nanoparticles are easy to detonate from the electrode, causing a micro-short circuit in the battery.
As far as we know, lithium iron phosphate batteries have a high short-circuit rate in both the manufacturing process of battery manufacturers and the use process of consumers. Battery manufacturers often start from the battery preparation process to find out the problem, but due to the intrinsic reason for lithium iron phosphate material caused by a short circuit, this problem is often not recognized. A123's 18650 lithium iron phosphate battery caught fire a few years ago on an electric car traveling on a highway. Investigations later concluded that faulty wiring was responsible for overheating and causing the battery to burst into flames. But we think a short circuit in the battery is more likely to cause a fire or an explosion. It is doubtful that the heat caused by the failure of the external screws to be tightened would have caused such a severe fire explosion in the 18650 lithium-ion battery.

Safety of Lithium Iron Phosphate Lithium-ion Battery
in Technology





0 comments