The development of lithium-ion batteries is the propeller of development progress in the modern world. People are keeping a high level of interest in how to improve battery performance, and researchers are constantly exploring various ways to optimize the performance of batteries. Whether it's creating the world's fastest electrodes, making battery components from nuclear waste, or preventing fire hazards with the help of sound waves, in 2020 scientists are showing us how imaginative they can be when it comes to developing the next generation of energy storage technology.
Researchers have explored many ways to improve electrode performance, for example, adding small amounts of graphene to make electrolytes more rigid, and how advanced materials can achieve fast battery charging or provide higher energy density. The foreign media Newatlas website selected the top ten technological breakthroughs in the field of batteries, the following, and lithium frontier editor to see the highlights of these studies and some disruptive, thinking outside the box battery design?


There are many options for improving battery performance through the introduction of new materials, one of which is lithium metal, which has great potential for development. Lithium metal has been described as a "dream material", using lithium metal as an anode to replace the graphite and copper currently used, can significantly improve the energy density of the battery, allowing the battery to work longer and store more energy.
But the biggest problem with lithium metal is safety. When the battery is charged, dendritic lithium dendrites can form on the surface of the lithium metal, which can lead to short circuits, fires, and eventually device failure. There are some new ways to solve this problem in 2020, one of which is Min-Kyu Song, a professor at Washington State University, whose method of preventing lithium dendrites from forming is to add a certain chemical to the cathode and electrolyte solution to form a protective layer on the surface of the lithium metal anode so that it remains stable over 500 charge and discharge cycles. It is worth mentioning that this process can be integrated into existing manufacturing procedures to facilitate mass production manufacturing.
Solid-state batteries without dendrites

This battery has an excellent energy density, about four times that of the Tesla Type 3 lithium battery, with a capacity of 1 kWh/liter. In terms of weight, this battery also provides 380 to 500 watt-hours/kg, exceeding the 260 watt-hours/kg of Tesla's battery pack. The study also found that this battery retains 80% of its capacity after 800 cycles, making it superior to other batteries in terms of battery life and safety.


While this design is comparable to conventional designs in terms of safety, this battery structure has also been shown to produce a larger current with this cell. It is so much larger than a conventional battery that the team reports that this device can handle five times the current of a conventional battery, which provides a research direction for a battery that can be fully charged for a short period.

In June, scientists at the Korea Institute of Science and Technology used a technique called lithium preloading to improve battery life, which involves immersing a silicon anode in a special solution that allows electrons and lithium ions to seep into the electrode to compensate for losses during cycling.
While most silicon-based anodes lose more than 20% of their lithium ions during the initial charge cycle, this new anode lost less than 1% during the test. It also has a 25% higher energy density than similar products on the market.

Purdue University scientists were able to reduce PET plastic from recyclable PET into flakes that were then treated with ultrafast microwave radiation to turn it into a substance known as disodium terephthalate, a small organic molecule with excellent electrochemical properties that have long been considered a potential anode material, which the team says is part of the sodium-ion battery assembly.
Lead researcher Vilas Boll said, "Stemming from society's concerns and growing awareness of climate change and energy resource constraints, we are addressing the proliferation of renewable energy conversion and storage."

Last June, a team of researchers at MIT demonstrated how the key building blocks of these batteries could be made from more sustainable materials. Chitin is a cellulose-like polysaccharide found in shrimp shells, and the researchers were able to use it in combination with felt to create electrodes for redox liquid flow batteries with higher energy densities.
Francisco Martínez, the senior author of the study, said, "Its benefits lie not only in its good performance but also in the low cost of the starting material and the consideration of reuse of waste, which makes the electrodes more sustainable value."

The energy is created in just 15 minutes, with a sustained power output of up to 8 hours and a peak output between 1 and 20 megawatts. This will be a low-cost, sustainable energy solution, and the company is ramping up construction of a prototype system, which will be ready for testing in Edinburgh by the end of 2021.

A research team at Brown University sought a solution to this problem by studying graphene materials, adding small amounts of graphene to ceramic materials to form a new solid electrolyte that they claim is the hardest yet. The novelty of this research is that graphene is highly conductive, which is not a good performance indicator for electrolytes, but they kept the concentration of graphene low enough to find a sweet spot that prevents it from conducting electricity and still provides a high degree of hardness.
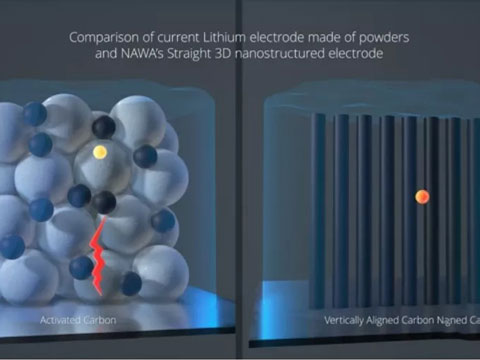
The electrode consists of a vertically aligned structure, similar to that of a comb, with 100 billion highly conductive carbon nanotubes standing upright on bolts and coated with an active material, such as lithium ions. This amounts to creating a highway for the moving ions, allowing them to move in and out of the battery more conveniently.
The company says its electrodes could increase the charging and discharging efficiency of the battery by a factor of 10, allowing for a 0-80% charge in five minutes. At the same time, energy density could jump by a factor of two to three.
Nawa says its process for producing these electrodes is inexpensive and is confident that it will be more cost-competitive with existing electrodes. The company expects the technology to enter the market starting in 2022 and to be able to develop more advanced technology systems after 2023. The company is currently in discussions with several automotive companies in this regard and has already found a major customer in France, Saft.








0 comments